EXPLORE MET BIOMARKERS IN NSCLC
*Estimated prevalence in non-squamous NSCLC. Imagery is for illustrative purposes only and does not
proportionally represent biomarker prevalence as shown.
Different MET Aberrations
c-Met protein overexpression presents on the surface of the tumor cells. MET gene aberrations, such as MET amplification and METex14 skipping, occur inside the tumor cells.5,8 There may be overlap among any or all of these MET biomarkers, and each has a role in the MET signaling pathway. There may also be overlap among MET biomarkers and other NSCLC biomarkers.2,7,12
c-Met protein
When its ligand hepatocyte growth factor (HGF) binds to and activates the c-Met receptor, there is an increase in cell motility, migration, and proliferation. This can lead to tumor growth and progression.2,8
MET amplification
MET amplification may occur in the setting of focal or regional gene duplication through breakage-fusion-bridge mechanisms.5 Increased copies of the MET gene can drive carcinogenesis.2
METex14 skipping mutations
Mutations that disrupt splice sites flanking METex14 result in aberrant splicing, which leads to METex14 skipping.5 This results in impaired c-Met degradation, which can activate MET signaling and promote tumorigenesis.2
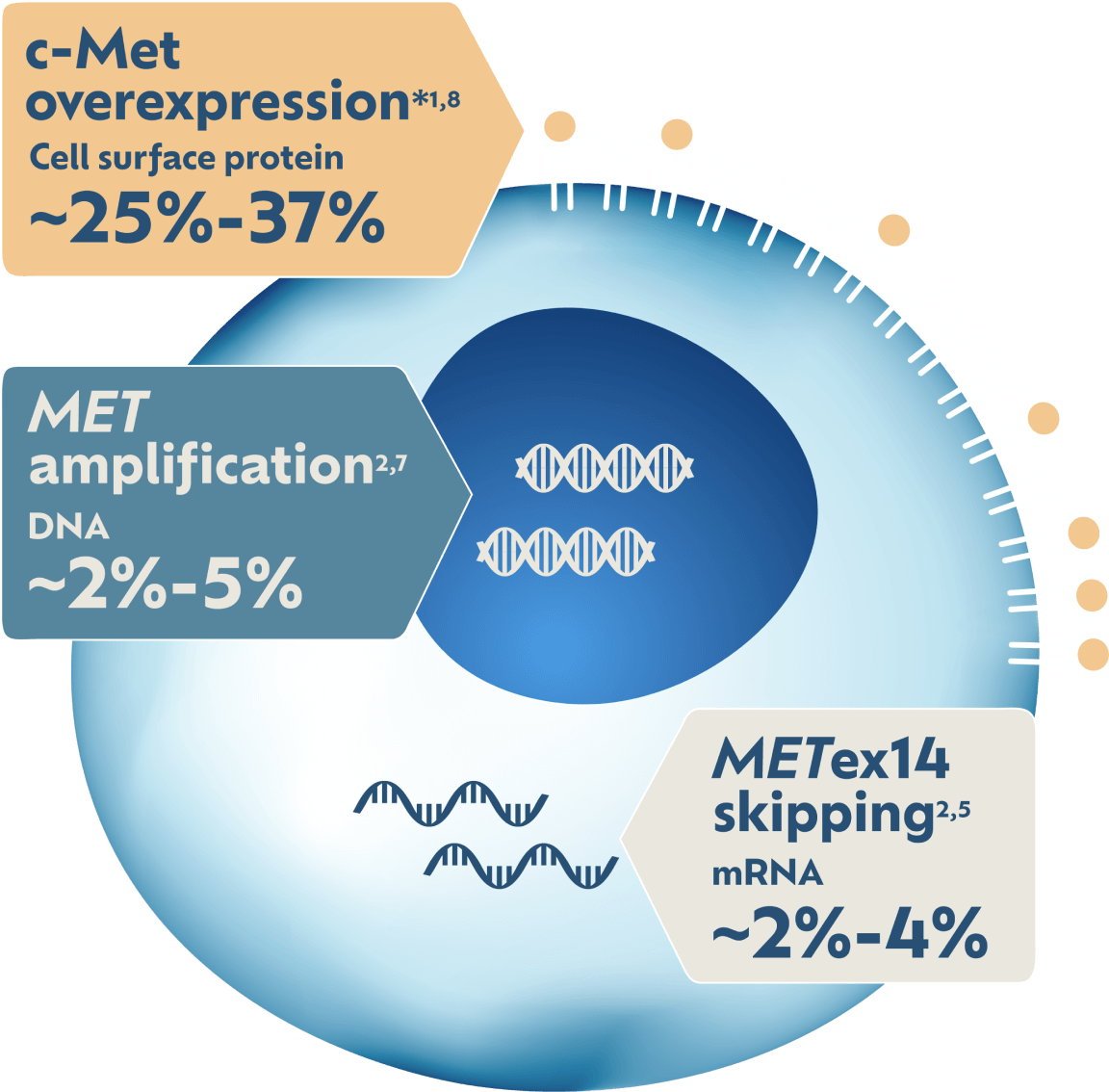
May Impact 5X More Patients1,2
c-Met protein overexpression has the highest prevalence of any MET biomarker in NSCLC: ~25%-37%.*1,2 MET amplification has ~2%-5% prevalence and METex14 skipping has ~2%-4% prevalence.2
*Estimated prevalence in non-squamous NSCLC. Biomarker prevalence estimates are based on multiple sources. Prevalence data can vary among studies and data sets because of detection methodology used and patient sample sizes and/or demographic characteristics. Some patients may have more than one MET aberration.
Exploring MET Detection Methods
IHC is an established testing method for assessing protein expression in NSCLC.1,7,9 NGS and FISH are examples of genetic testing modalities that can be used to detect amplification and/or exon skipping mutations.5,7
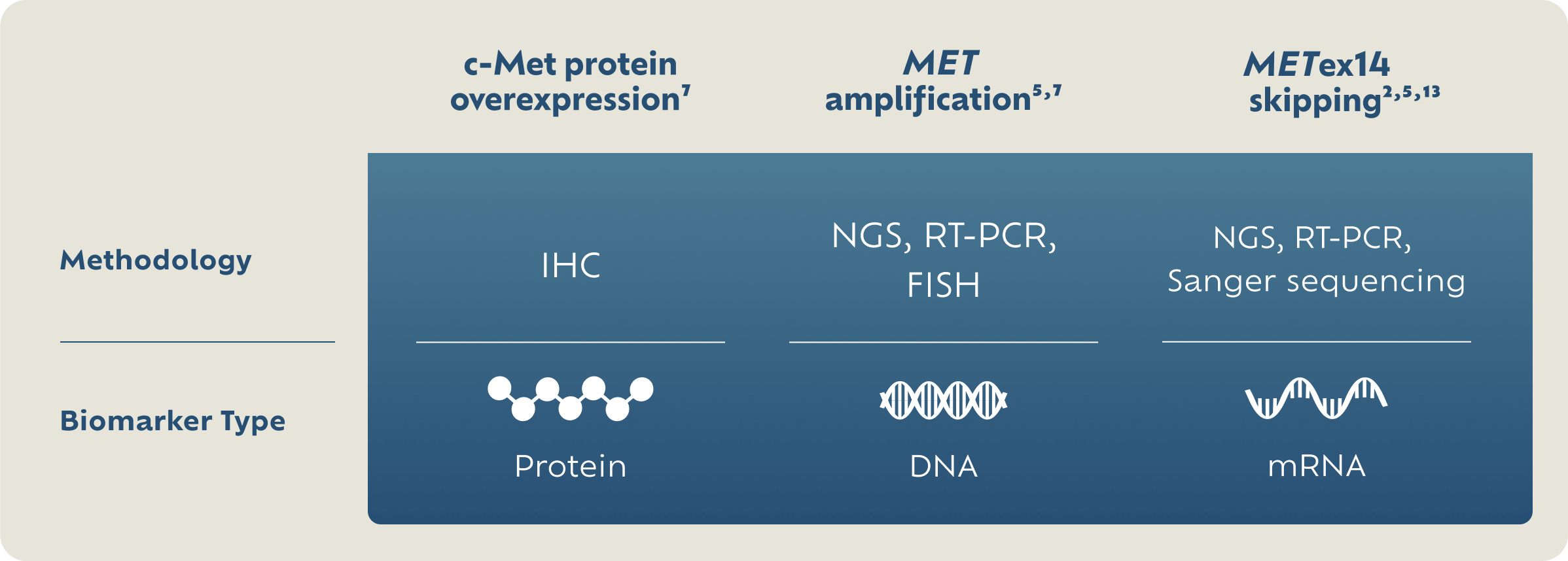
There are no FDA-approved tests for c-Met protein overexpression or MET gene amplification.
FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NGS, next-generation sequencing; RT-PCR, reverse transcription-polymerase chain reaction.
c-Met protein overexpression and MET gene amplification are emerging biomarkers in clinical research as potential therapeutic targets.
There are no FDA-approved tests for c-Met protein overexpression or MET gene amplification.
Biomarkers included are select MET aberrations that are both emerging and established biomarkers in NSCLC. This is not an exhaustive list of all NSCLC biomarkers and selected MET aberrations may overlap with other NSCLC biomarkers.
MET aberration prevalence estimates and prognosis statements are based on multiple sources. Survival and prevalence data can vary among studies and data sets because of detection methodology used, patient sample sizes, and/or demographic characteristics. Some patients may have more than one MET aberration.
