*Estimated prevalence in non-squamous NSCLC.
Imagery is for illustrative purposes only and does not proportionally represent biomarker prevalence as shown.
c-Met protein overexpression and MET gene amplification are emerging biomarkers in clinical research as potential therapeutic targets.
*Estimated prevalence in non-squamous NSCLC.
Imagery is for illustrative purposes only and does not proportionally represent biomarker prevalence as shown.
Ongoing Challenges in 2L NSCLC
Prognosis remains poor for certain NSCLC patients in the 2L setting, with limited options. It is critical to identify more NSCLC biomarkers to expand our understanding of advanced disease and explore potential opportunities to enhance patient care.2-4
Current and Emerging MET Biomarkers
Several MET aberrations exist. There are gene biomarkers such as METex14 skipping and MET amplification.5,6 There is also an emerging cell surface protein biomarker in research called c-Met protein overexpression.1,3,7 Learn more about these MET biomarkers.
Prevalence
c-Met protein overexpression occurs in ~25%-37% of patients with NSCLC.*1 ~2%-5% of patients have MET amplification and ~2%-4% have METex14 skipping.2
*Estimated prevalence in non-squamous NSCLC.
Biomarker prevalence estimates are based on multiple sources. Prevalence data can vary among studies and data sets because of detection methodology used and patient sample sizes and/or demographic characteristics. Some patients may have more than one MET aberration.

Different MET Aberrations
c-Met is a cell surface protein. MET amplification and METex14 skipping are genetic aberrations inside the cell.5,8
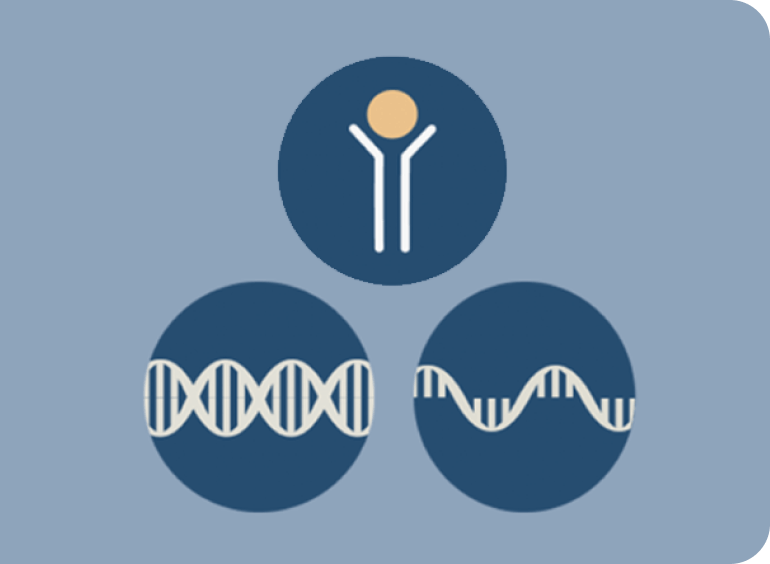
Testing Methodologies
IHC is an established testing method for assessing protein expression such as c-Met.1,7,9 NGS and FISH are examples of genetic testing modalities for METex14 skipping and MET amplification.5,7
There are no FDA-approved tests for c-Met protein overexpression or MET gene amplification.
FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NGS, next-generation sequencing.

Poorer Prognosis in NSCLC
Patients with MET aberrations may face poorer prognosis in NSCLC.6,10 Ongoing research continues to explore the prognostic value of MET aberrations.1,3,7,10,11
MET aberration prognosis statements are based on multiple sources. Survival data can vary among studies and data sets because of detection methodology used and patient sample sizes and/or demographic characteristics. Some patients may have more than one MET aberration.

c-Met protein overexpression and MET gene amplification are emerging biomarkers in clinical research as potential therapeutic targets.
There are no FDA-approved tests for c-Met protein overexpression or MET gene amplification.
Biomarkers included are select MET aberrations that are both emerging and established biomarkers in NSCLC. This is not an exhaustive list of all NSCLC biomarkers and selected MET aberrations may overlap with other NSCLC biomarkers.
MET aberration prevalence estimates and prognosis statements are based on multiple sources. Survival and prevalence data can vary among studies and data sets because of detection methodology used, patient sample sizes, and/or demographic characteristics. Some patients may have more than one MET aberration.
